Bio X Cell社 – In Vivo Depletion of Mouse Neutrophils Using Anti-Ly6G Antibodies
Neutrophils are the most abundant population of white blood cells in circulation, accounting for approximately 60-70% of adult human peripheral leukocytes and 10-25% in mice. Neutrophils exhibit a high degree of heterogeneity and plasticity and play a variety of seemingly contradictive roles, protective versus pathogenic. Neutrophils are important effector cells in antibacterial defense, destroying pathogens through phagocytosis, degranulation, and formation of neutrophil extracellular traps (NETs), while they have critical functions related to tissue repair and fibrosis [1].
Neutrophils also show a multifaceted nature in the tumor microenvironment, where they release toxic substances such as reactive oxygen species and metalloproteinases, modulate tumor necrosis factor-related ligands to induce tumor cell apoptosis, and activate a network of immune cells to enhance antitumor effects. However, neutrophils themselves at the same time undergo reprogramming that promotes immune evasion, angiogenesis, and tumor growth [2,3]. The diverse and complicated roles of neutrophils have emerged as a prominent area of immunological research in recent years.
Figure 1 Number of citations returned with “neutrophil” in PubMed as of December 31, 2024, with a surge since 2020.
The short lifespan and rapid infiltration of neutrophils used to be a technical challenge, yet recent advancement of research tools has bolstered our understanding of neutrophils. To explore in vivo whether it is neutrophils at work, an extensively used approach is to inject experimental animals, such as mice, with anti-Ly6G antibodies, which deplete neutrophils in vivo, and then conduct functional assays, for example, measuring tumor size upon neutrophil depletion.
Mouse Ly6G is a glycosylated phosphatidylinositol-anchored cell-surface protein, typically expressed transiently during monocyte development and persistently in mature granulocytes and peripheral neutrophils. Its 1A8 monoclonal antibodies react specifically with mouse Ly6G, and it is widely used for in vivo neutrophil depletion [4].
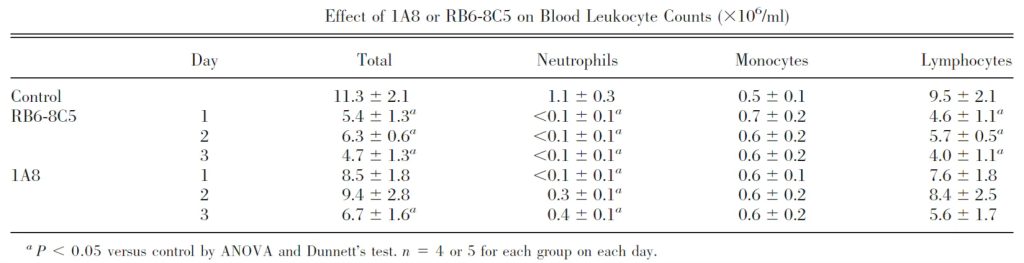
Table 1 Anti-Ly6G antibody 1A8 depletes neutrophils in mice more specifically than anti-Gr-1 antibody RB6-8C5 with less effect on blood lymphocytes [4].
According to the CiteAb, two anti-mouse Ly6G antibody clones 1A8 from Bio X Cell, InVivoMAb anti-mouse Ly6G (#BE0075-1) and InVivoPlus anti-mouse Ly6G (#BP0075-1), have been cited in 296 and 88 publications, respectively, as of the end of 2024, and have shown quite nice effects in vivo. Both products are ultra-pure and 0.2µm filtered to eliminate the influence of endotoxin and azide. The InVivoPlus version contains even lower endotoxin of less than 1EU/mg (<2EU/mg for InVivoMAb) and meets additional strict demands for binding validation, murine pathogen screening and antibody aggregation screening.
| PMID | Title | Journal (Year) |
| 39385035 | CTLA4 blockade abrogates KEAP1/STK11-related resistance to PD-(L)1 inhibitors | Nature (2024) |
| 39143217 | Recognition and control of neutrophil extracellular trap formation by MICL | Nature (2024) |
| 39112714 | DNA-sensing inflammasomes cause recurrent atherosclerotic stroke | Nature (2024) |
| 38838669 | Clonal hematopoiesis driven by mutated DNMT3A promotes inflammatory bone loss | Cell (2024) |
| 38518773 | Biofilm exopolysaccharides alter sensory-neuron-mediated sickness during lung infection | Cell (2024) |
| 38447573 | Neutrophil profiling illuminates anti-tumor antigen-presenting potency | Cell (2024) |
| 38428422 | Maternal inflammation regulates fetal emergency myelopoiesis | Cell (2024) |
| 38029747 | Early cellular mechanisms of type I interferon-driven susceptibility to tuberculosis | Cell (2023) |
| 38016470 | Prolonged sleep deprivation induces a cytokine-storm-like syndrome in mammals | Cell (2023) |
| 37674079 | CD300ld on neutrophils is required for tumour-driven immune suppression | Nature (2023) |
| 37316667 | CD4+ T cell-induced inflammatory cell death controls immune-evasive tumours | Nature (2023) |
| 37258670 | In situ tumour arrays reveal early environmental control of cancer immunity | Nature (2023) |
| 37001504 | A neutrophil response linked to tumor control in immunotherapy | Cell (2023) |
| 37001503 | T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants | Cell (2023) |
Table 2 Bio X Cell mouse Ly6G antibodies (clone 1A8) selected citations of in vivo applications
Here three high-impact publications in 2024 are selected to briefly introduce application of anti-Ly6G antibodies for neutrophil depletion in vivo and the key findings.
Study case I
CD49f-high hepatocellular carcinoma cells promote immune evasion [5]
A Cancer Cell paper from the joint team of Renji Hospital-Shanghai Institute of Cancer Research, School of Medicine, Shanghai Jiao Tong University, and Zhongshan Hospital, Fudan University.
The authors first identified CD49f as a prominent marker of tumor-initiating cells (TICs) in hepatocellular carcinoma, and found that CD49f-high TICs recruit neutrophils via the CXCL2-CXCR2 axis to create an immunosuppressive microenvironment.
Subsequently, to determine whether neutrophils exert reciprocal effects on the TIC phenotype, the authors constructed orthotopic HCC tumors using Hep53.4-GFP cells, followed by treatment with InVivoMAb anti-mouse Ly6G antibody or an equivalent dose of isotype control InVivoMAb rat IgG2a (#BE0089) at either 10mg/kg or 7.5mg/kg. The results showed that anti-Ly6G antibody successfully depleted neutrophils and significantly impaired the tumorigenic potential of GFP+ cells compared to the control group, as well as reduced proportion of CD49f+ tumor cells in the tumor, indicating that neutrophils are also crucial for maintaining the TIC phenotype.
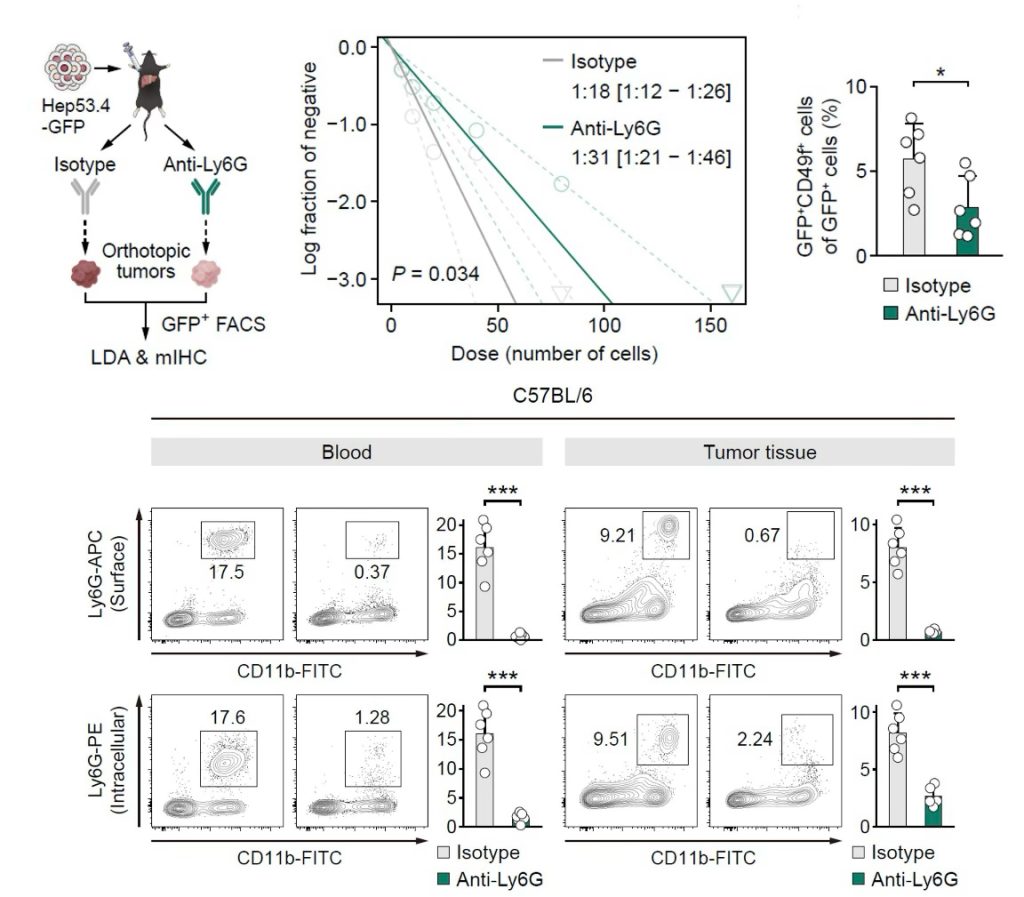
Figure 2 Upper row from left to right, schematic illustrating the process of determining whether neutrophils influence tumorigenicity, extreme limiting dilution analysis measuring the tumorigenic potential of Hep53.4 cells, and abundance of CD49f+GFP+ cells. Lower row, flow cytometry analysis of blood or tumor tissues to validate neutrophil depletion efficiency in C57BL/6 mice, n=6 mice/group.
Study case II
Control of neutrophil extracellular trap formation by MICL [6]
The study, published in Nature, found that myeloid-inhibitory C-type lectin-like receptor (MICL) functions as an essential pattern recognition receptor for NETs, and this interaction inhibits neutrophil activation and further NET formation. High levels of autoantibodies to MICL link to the progression of autoimmune diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus.
The authors constructed a CAIA (collagen antibody-induced arthritis) model of RA using Micl-/- mice, which exhibit enhanced and non-resolving arthritic phenotypes. Flow cytometry analysis further revealed a specific increase and heightened activation phenotype of neutrophils in the joints of Micl-/- animals.
To investigate whether neutrophils were solely responsible for the exacerbated disease in Micl-/- mice, the authors successfully depleted circulating neutrophils via administering of 500µg of anti-Ly6G antibody (clone 1A8, Bio X Cell) or rat IgG2a isotype control to mice every 48 hours from day 5 after injection of the ArthritoMab monoclonal antibody cocktail. Notably, wild type and Micl-/- mice showed a significant and equivalent reduction in clinical disease following neutrophil depletion, indicating that the elevated pathology in the Micl-/- mice is stemming from neutrophils.
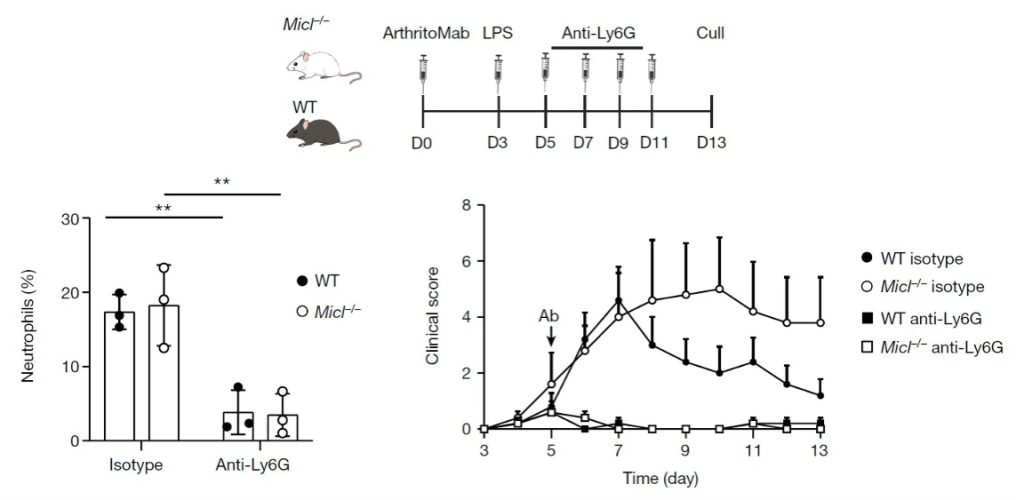
Figure 3 Upper panel, schematic representation of the anti-Ly6G-mediated neutrophil depletion strategy in the CAIA model. Lower left, flow cytometry quantification of neutrophils (CD45+CD11b+F4/80-SSChigh) in the peripheral blood on day 9 (n=1 experiment with 3 mice per group). Lower right, severity scores of mice (n=1 experiment with 5 mice per group).
Study case III
Critical role of neutrophil-derived migrasome in coagulation[7]
A recent Nature Cell Biology paper from Tsinghua University reported a new function of migrasomes in coagulation. Migrasomes are organelles generated by migrating cells.
The authors found that a large number of neutrophil-derived migrasomes exist in the blood of mice and humans. These migrasomes adsorb and enrich coagulation factors on their surfaces and are highly enriched with adhesion molecules, which enable them to preferentially accumulate at the site of injury, activate platelets and trigger coagulation.
Next, to test the role of neutrophil-derived migrasomes in coagulation in vivo, mice were injected intraperitoneally InVivoPlus anti-Ly6G antibody and the isotype control InVivoPlus rat IgG2a (#BP0089), at an initial dose of 200µg, followed by 100µg three times per week. Flow cytometry analysis confirmed that vast majority of neutrophils were depleted by anti-Ly6G antibody treatment, whereas the number of platelets was not affected. Depletion of neutrophils significantly increased the bleeding volume in the tail tip bleeding assay, whereas injection of purified neutrophil-derived migrasomes rescued impaired coagulation in neutrophil-depleted mice, suggesting that neutrophil-derived migrasomes play an essential role in coagulation in vivo.
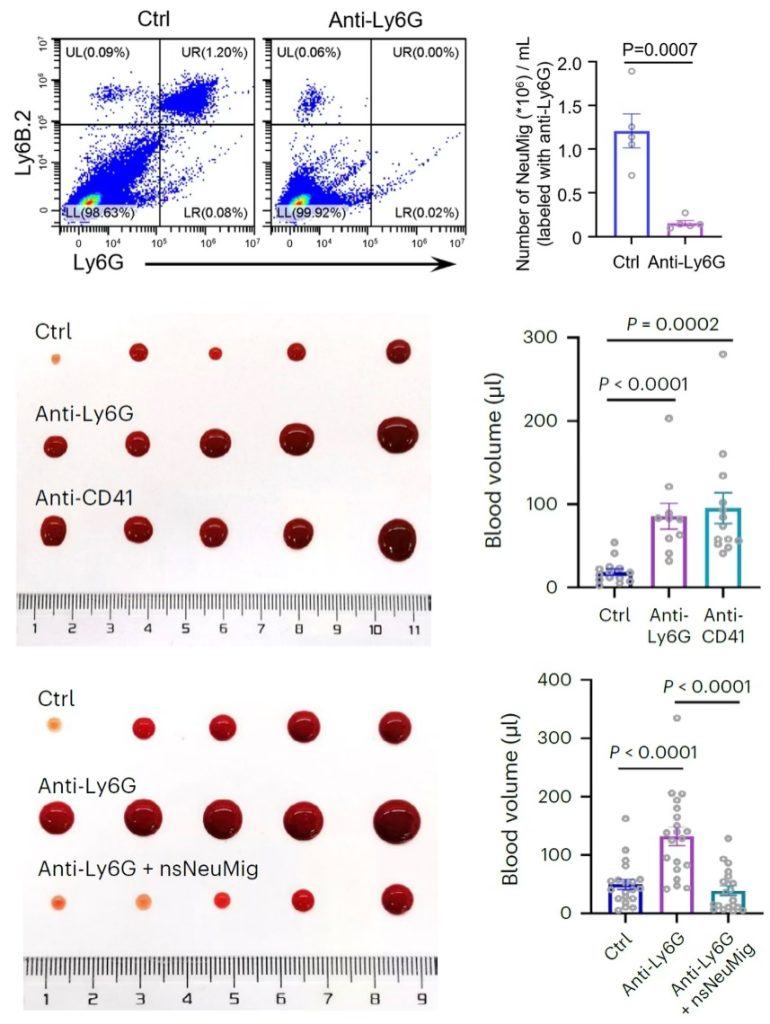
Figure 4 Upper row, flow cytometry analysis of mouse whole blood cells, and quantification of the number of neutrophil-derived migrasomes revealed by imaging-flow cytometry analysis. Middle row, tail tip bleeding assay in control, neutrophil-depleted (anti-Ly6G) and platelet-depleted (anti-CD41) mice. Lower row, tail tip bleeding assay in control, neutrophil-depleted (anti-Ly6G) mice and mice injected with neutrophil-derived migrasomes.
Notes for neutrophil depletion using anti-Ly6G antibodies
To determine the appropriate dose and frequency for antibody administration in a specific experiment, quite a few factors need to be considered, such as mouse strain, age, body weight, disease model, study duration, and environmental factors, etc. A single dose of clone 1A8 for neutrophil depletion usually ranges from 7.5mg/kg to 20mg/kg. Please refer to extensive citations of Bio X Cell’s products and conduct pre-tests.
Meanwhile, isotype control is required to eliminate background, and flow cytometry analysis should be performed to measure depletion efficiency in vivo. Bio X Cell offers isotype controls for each of the two versions of anti-Ly6G antibodies, InVivoMAb rat IgG2a isotype control (#BE0089) and InVivoPlus rat IgG2a isotype control (#BP0089).
In addition to these general principles of in vivo antibody application, in terms of neutrophil depletion using anti-Ly6G antibodies, it may have limited efficacy particularly in depleting immature neutrophils. For instance, a Cell paper in 2023 reported that 28% of neutrophils remained in MC38 tumors after anti-Ly6G antibody treatment, and the persisting cells were less mature, indicated by their lower expression of CD101 and CXCR2, as well as higher expression of CXCR4 [8]. In contrast, in a back-to-back Cell paper, neutrophils were effectively depleted using anti-Ly6G antibody probably due to a more mature anti-tumorigenic phenotype in immunotherapy-treated mice [9].
Moreover, a study in Science Immunology in 2024 identified an atypical population of Ly6G-expressing macrophages based on a model of influenza A virus-induced lung injury, breaking the usual perception that Ly6G expression only persists in mature granulocytes [10].
In vitro application of Bio X Cell anti-Ly6G antibody
In addition to in vivo depletion, anti-mouse Ly6G clone 1A8 is also widely used in flow cytometry, immunohistochemistry (IHC), immunofluorescence (IF) and other in vitro analysis experiments. In fact, several studies have been conducted using Bio X Cell’s 1A8 clones for indirect IHC and IF, which is more cost-effective compared to small package antibodies designed for in vitro applications. These results also provide evidence for the specificity and sensitivity of functional antibodies.
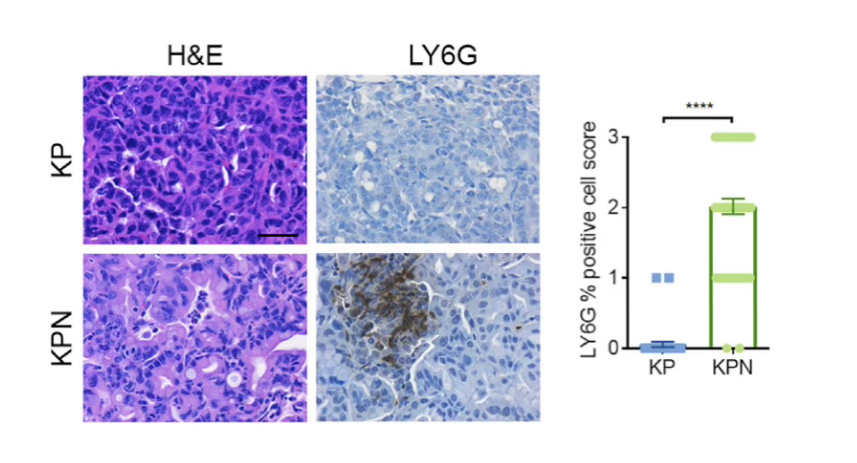
Figure 5 IHC images and quantification of positive cells in FFPE sections of KrasLSL-G12D/+;Trp53fl/fl (KP) and KrasFSF-G12D/+;Trp53Frt/Frt;Rosa26FSF-CreERT2;Nkx2-1fl/fl (KPN) mouse tumors using 1:1500 dilution of InVivoMAb anti-mouse Ly6G [11].
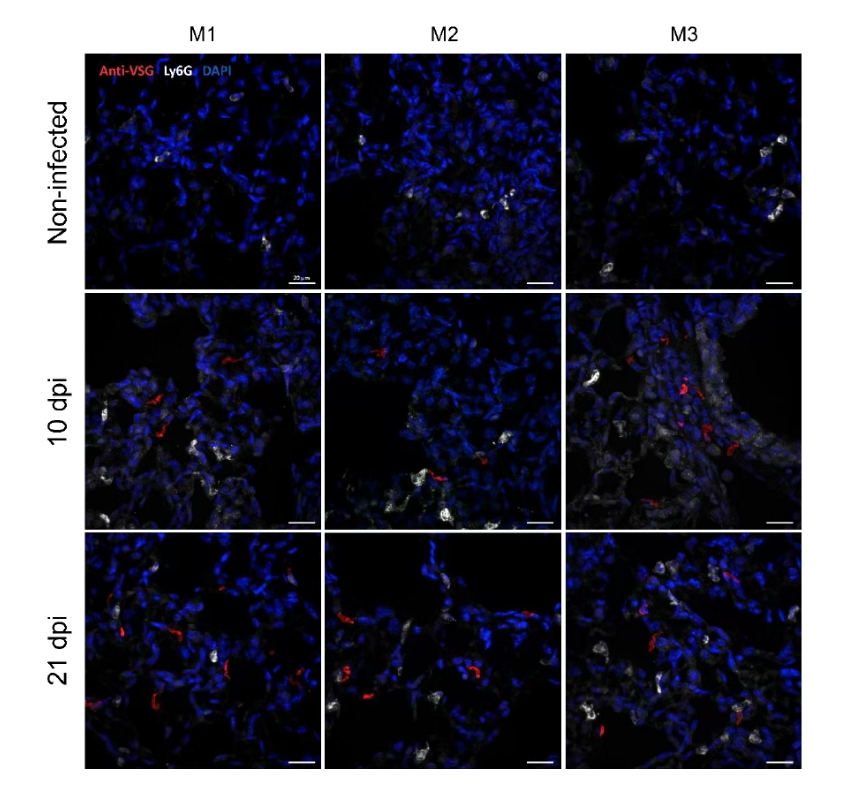
Figure 6 IF in parasite-infected mouse lung tissue frozen section using 1:2000 dilution of InVivoMAb anti-mouse Ly6G [12].
1. Herro, R, et al (2024) The diverse roles of neutrophils from protection to pathogenesis. Nat Immunol. 25(12):2209-2219. doi: 10.1038/s41590-024-02006-5.
2. Huang, X, et al (2024) Neutrophils in cancer immunotherapy: friends or foes? Mol Cancer. 23(1):107. doi: 10.1186/s12943-024-02004-z.
3. Ng, M, et al (2025) Adaptations of neutrophils in cancer. Immunity. 58(1):40-58. doi: 10.1016/j.immuni.2024.12.009.
4. Daley, JM, et al (2008) Use of Ly6G-specific nonoclonal antibody to deplete neutrophil in mice. J Leukoc Biol. 83(1):64-70. doi: 10.1189/jlb.0407247.
5. Yang, C, et al (2024) Targeting the immune privilege of tumor-initiating cells to enhance cancer immunotherapy. Cancer Cell. 42(12):2064-2081. doi: 10.1016/j.ccell.2024.10.008.
6. Malamud, M, et al (2024) Recognition and control of neutrophil extracellular trap formation by MICL. Nature. 633(8029):442-450. doi: 10.1038/s41586-024-07820-3.
7. Jiang, D, et al (2024) Neutrophil-derived migrasomes are an essential part of the coagulation system. Nat Cell Biol. 26(7):1110-1123. doi: 10.1038/s41556-024-01440-9.
8. Gungabeesoon, J, et al (2023) A neutrophil response linked to tumor control in immunotherapy. Cell. 186(7):1448-1464. doi: 10.1016/j.cell.2023.02.032.
9. Hirschhorn, D, et al (2023) T cell immunotherapies engage neutrophils to eliminate tumor antigen escape variants. Cell. 186(7):1432-1447. doi: 10.1016/j.cell.2023.03.007.
10. Ruscitti, C, et al (2024) Recruited atypical Ly6G+ macrophages license alveolar regeneration after lung injury. Sci Immunol. 9(98):eado1227. doi: 10.1126/sciimmunol.ado1227.
11. Mollaoglu, G, et al (2018) The lineage-defining transcription factors SOX2 and NKX2-1 determine lung cancer cell fate and shape the tumor immune microenvironment. Immunity. 49(4):764-779. doi: 10.1016/j.immuni.2018.09.020.
12. Mabille, D, et al (2022) Impact of pulmonary African trypanosomes on the immunology and function of the lung. Nat Commun. 13(1):7083. doi: 10.1038/s41467-022-34757-w.
各商品の価格のお問合せ、商品仕様書のご依頼、その他のお問い合わせは下記までお願いします。
【商品取扱元】株式会社 東京未来スタイル
info@tokyofuturestyle.com
TEL:029-851-9222 FAX:029-851-9220

